What is molecular biotechnology?
Molecular biotechnology is an interdisciplinary field at the interface of biology, chemistry and engineering. It uses molecular and genetic tools, such as DNA cloning, PCR, and recombinant DNA technology, to study and manipulate biomolecules (DNA, RNA, proteins) for practical ends. In simple terms, molecular biotechnology harnesses living cells and their components to develop new products and technologies that improve human health, agriculture and the environment. For example, by modifying nucleic acids and proteins, molecular biotechnologists create medicines, vaccines, diagnostic tests or biofuels in ways that were impossible just a few decades ago.
Unlike general biotechnology, which can include large-scale processes like fermentation, molecular biotechnology focuses on the molecular details. It combines techniques from molecular biology (studying DNA and RNA) and biochemistry to engineer cells or enzymes at the genetic level. For instance, scientists might insert a human gene into bacteria so the microbe produces a human protein (such as insulin), a classic molecular biotechnology application. The goal is often to translate molecular knowledge into real-world products: medicines, better crops, safer foods, or novel materials.
How does molecular biotechnology differ from molecular biology and biophysics?
Molecular biology is a fundamental science that investigates how biological molecules (like genes and proteins) function in cells. It answers questions such as how DNA is copied, how genes are turned on or off, and how proteins are built from genes. Molecular biologists use lab experiments and computational tools to understand these basic processes.
By contrast, molecular biotechnology applies that molecular knowledge for practical uses. In other words, molecular biology provides the knowledge of how biomolecules work, and molecular biotechnology uses that knowledge to engineer new products or processes. As one source explains, molecular biology “studies microorganisms and the effects they have on people’s lives,” whereas biotechnology “uses this knowledge to develop technologies and processes” that solve problems like disease or food shortages. In practice, a molecular biologist might study how a virus infects cells, while a molecular biotechnologist could use that information to design a new antiviral drug or a viral vaccine.
Biophysics is another related field that intersects with molecular biotechnology. Biophysics brings the tools of physics and chemistry into biology, it focuses on the physical forces and structures within biological molecules. For example, biophysicists might use X-ray crystallography or NMR to determine a protein’s 3D shape, or measure the forces that cause a molecular machine to change shape during a reaction. In essence, biophysics seeks to explain biological function in terms of physics (structure, energy, motion). While biophysics is mostly about understanding nature, molecular biotechnology is about changing or using nature.
A biophysicist might describe how a protein channel opens and closes, whereas a biotechnologist might engineer a synthetic version of that channel for a biosensor. In summary, biophysics provides mechanistic insight at the molecular level, and molecular biotechnology uses that insight (and other molecular biology tools) for design and engineering.

What does a molecular biotechnologist do?
A molecular biotechnologist designs and performs experiments at the molecular level. In practice, they work in a lab using techniques like DNA sequencing, PCR, gene cloning, and cell culture to answer questions or create products.
For example, a molecular biotechnologist might insert a gene into bacteria so the microbe makes a useful protein, or they might analyze a patient’s DNA to find mutations linked to disease. Key duties include analyzing genetic material, developing new drugs or vaccines, and creating diagnostic tests. According to educational sources, typical responsibilities are developing better medicines, studying human and animal DNA, and researching vaccines. They may also work on agricultural or environmental projects, such as improving crop traits or engineering organisms to clean up pollutants.
What is an example of molecular biotechnology?
A classic example is the production of recombinant insulin. Scientists take the human insulin gene and insert it into bacteria. These engineered bacteria then produce human insulin protein, which is purified and used to treat diabetes.
This process, modifying DNA to manufacture a human protein, is a direct application of molecular biotechnology. Other examples include engineering yeast to make biofuels, creating genetically modified crops that resist pests or tolerate harsh conditions, and using CRISPR gene editing to create disease-resistant animals. Even PCR tests for detecting viruses (e.g. COVID-19 tests) are an example, since they rely on molecular biotech tools to amplify and read DNA/RNA. In short, any technology that uses genetic modification or molecular analysis for a tangible product, like a drug, vaccine, or improved plant variety, is an example of molecular biotechnology.
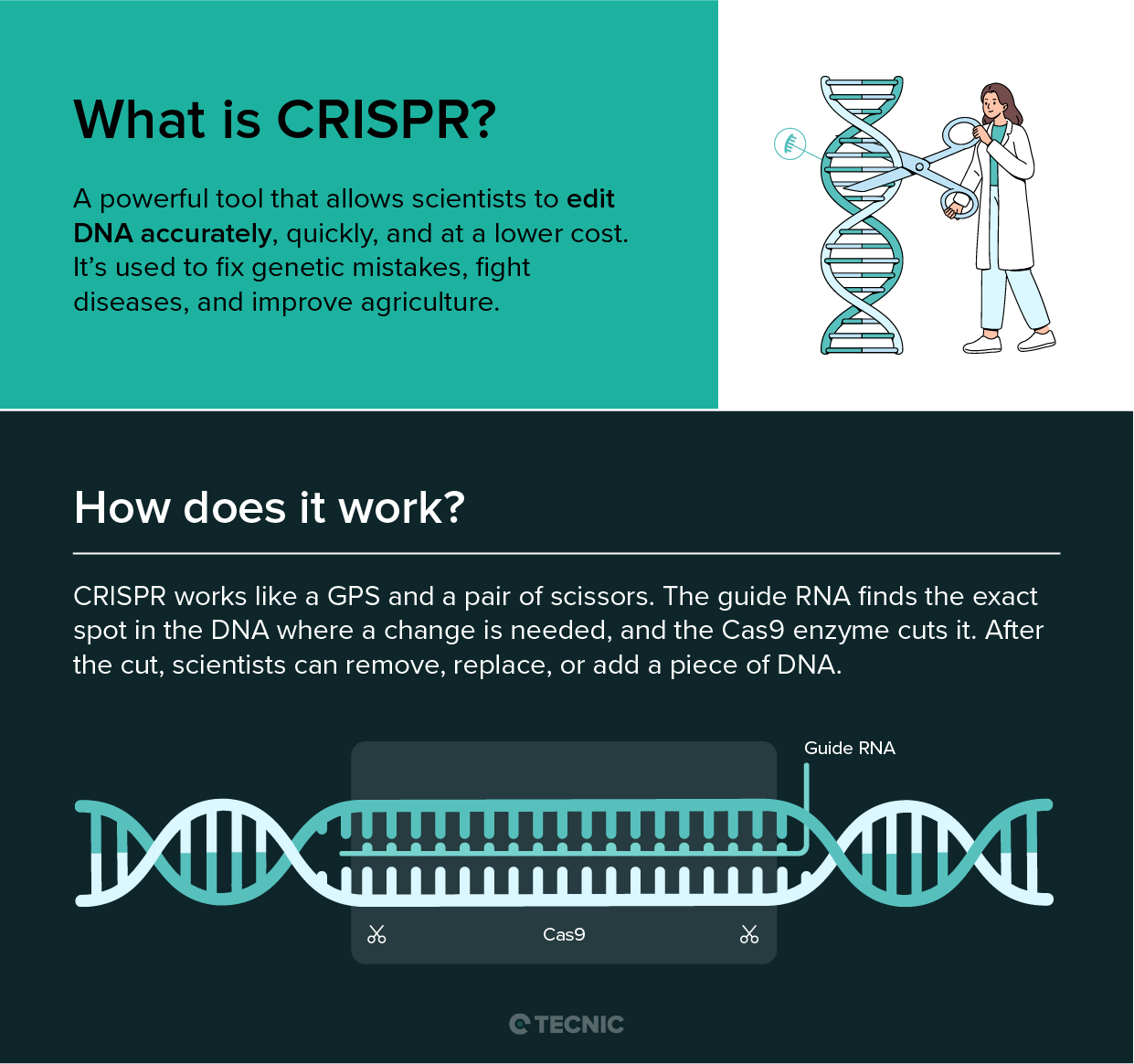
What are the applications of molecular biotechnology?
Molecular biotechnology touches many aspects of everyday life. In healthcare, it has revolutionized diagnostics and treatments. For example, molecular biotech techniques made modern vaccines and drugs possible: recombinant DNA is used to produce therapeutic insulin, monoclonal antibody drugs, and the mRNA vaccines against COVID-19.
Techniques like PCR and next-generation sequencing allow rapid diagnosis of infections (such as identifying viruses by their DNA/RNA) and personalized cancer therapy (by sequencing tumor DNA to find targetable mutations). Gene-editing tools like CRISPR/Cas9, a hallmark of molecular biotechnology, are now being developed into treatments that can correct genetic diseases or engineer immune cells to fight cancer. In the lab, biotechnologists routinely manipulate DNA/RNA in simple organisms (like bacteria or yeast) to produce human proteins, vaccines, enzymes or other therapeutic molecules at scale.
- Healthcare: Molecular biotechnology is used to develop new medicines, vaccines, and diagnostics. For instance, genetically engineered bacteria produce human insulin and growth hormone, cell cultures produce vaccines, and gene therapies aim to cure inherited diseases. Researchers also use molecular tools to create better diagnostic tests (PCR tests, DNA chips) and targeted therapies (such as CAR-T cell therapy for cancer).
- Agriculture and Food: In agriculture, molecular biotechnology creates improved crops and livestock. Examples include genetically modified (GM) crops that resist pests, tolerate drought, or have enhanced nutrition. Scientists can insert a gene for pest resistance into corn or engineer rice to produce vitamin-enriched grain. Biotech crops like “Golden Rice” (engineered to produce vitamin A precursor) help address nutritional deficiencies. Molecular tools also improve plant breeding by identifying beneficial genes faster (marker-assisted selection). In animal agriculture, biotechnology is used for disease-resistant animals and for producing vaccines for livestock. More broadly, molecular biotech has found roles in aquaculture (fish farming), food processing (using enzymes to make cheese or bread), and even biodegradable plastics derived from engineered microbes.
- Industrial and Environmental: Molecular biotechnology underlies many industrial processes. For example, engineered microbes produce biofuels (ethanol, biodiesel) from plant biomass more efficiently. Custom enzymes made by molecular biotech catalyze chemical reactions in manufacturing (such as enzymes in laundry detergent or in textile processing). It is also used in environmental cleanup: bacteria are engineered to break down oil spills or toxic waste through bioremediation. In the food and biotechnology industries, molecular biotech is used in fermentation, bioreactor production of biomolecules, and even the emerging field of synthetic biology (designing entire new biological systems). In short, molecular biotechnology enables “green” chemistry and bio-based manufacturing by leveraging the power of living cells.

What are emerging trends and research directions in molecular biotechnology?
Molecular biotechnology is advancing rapidly, driven by new tools and global challenges. Key emerging trends include:
- Gene Editing and Synthetic Biology: Techniques like CRISPR/Cas9, TALENs, and base editors allow precise editing of genomes. Researchers are not only editing genes but also constructing synthetic genetic circuits and even whole new organisms. This “synthetic biology” approach lets scientists build bacteria or yeast with custom functions (e.g. producing drugs or materials). As one review notes, synthetic biology now enables designing artificial cells and new gene combinations with desired properties. Ethical and regulatory debates are ongoing, but the technology is becoming faster, cheaper and more powerful every year.
- Personalized and Precision Medicine: Thanks to molecular biotechnology, medicine is becoming more individualized. Advances in DNA sequencing and “omics” technologies let doctors tailor treatments to a patient’s genetic profile. For example, genomics-guided cancer therapies target specific mutations, and pharmacogenomics helps choose drugs that work best for a person’s DNA. Cell therapies are part of this trend: engineered immune cells (CAR-T cells) have recently been approved for certain cancers, and dozens of personalized gene therapies are in trials. Overall, biotechnology research is focused on predictive diagnostics and therapies tuned to each person.
- Artificial Intelligence and Big Data: Modern biotechnology generates huge amounts of data (genomes, protein structures, etc.), and researchers increasingly use AI to make sense of it. Machine learning models now predict protein folding or identify drug candidates, accelerating discovery. Industry surveys report that most biopharma companies are prioritizing generative AI and data-driven R&D. AI is also used in laboratory automation (robotic experiments) and analyzing medical images. The integration of computational biology with molecular techniques is a hot research area, enabling faster design of experiments and in silico modeling of biological systems.
- Sustainable and Green Biotech: Environmental concerns are pushing research in bio-based solutions. This includes engineering microbes to produce renewable fuels, biodegradable plastics, or to capture carbon. New enzymes that degrade pollutants (xenobiotic-degrading enzymes) are being developed for cleaning up oil spills and chemical waste. Additionally, lab practices themselves are becoming greener, with an emphasis on reducing lab waste and energy use. On the production side, biomanufacturing (using cells to make chemicals) is trending, as companies seek sustainable manufacturing processes.
These trends are interconnected. For example, synthetic biology often relies on AI-driven design, and gene editing can be applied toward sustainable agriculture or medicine. The field is dynamic, researchers are exploring everything from lab-grown meat to personalized vaccines, and what is “emerging” today often becomes standard biotech practice in a few years.
What career paths and educational backgrounds are common in molecular biotechnology?
Molecular biotechnology is a broad field, and career paths are diverse.
Education: Most positions require at least a bachelor’s degree in a life science (molecular biology, biotechnology, biochemistry, or related fields). Undergraduate courses typically cover genetics, microbiology, and laboratory techniques. For research or specialized roles, a Master’s or Ph.D. in molecular biology or biotechnology is often preferred. Advanced degrees open doors to leading R&D and academic careers.
Job Roles: Common roles include Research Scientist (in industry or academia), Laboratory Technician or Technologist (supporting experiments and quality control), and Bioprocess/Biomanufacturing Engineer (scaling up production of biotech products). Biotech companies also hire Bioinformatics Specialists to analyze genetic data, Quality Control/Regulatory Affairs Specialists to ensure products meet standards, and Clinical Research Managers for biomedical trials. As one program description notes, graduates can work in the pharmaceutical or food industries, biotech companies, or in laboratory analysis. Typical workplaces range from industrial R&D labs and hospital labs to environmental agencies and agricultural firms.
For example, a molecular biotechnologist might work in a pharmaceutical company developing new drugs, in an agricultural biotech firm designing pest-resistant crops, or in a diagnostic lab using PCR to detect pathogens. Smaller biotech startups, government research institutes, and university labs also hire molecular biotechnologists. The field also includes emerging roles like Synthetic Biology Engineer or Genomics Data Scientist. Because the biotechnology industry intersects with chemistry, engineering, and computer science, people with interdisciplinary training (e.g. bioengineering or computational biology) are in demand. Overall, the career outlook is positive: the biotechnology sector is growing and offers diverse opportunities for those with molecular science training.

What career paths and educational backgrounds are common in molecular biotechnology?
Salaries in molecular biology and biotechnology depend on position, location, and experience. While entry-level positions can start around $35,000-45,000, the median annual salary for biological scientists in the U.S. was about $66,400 in 2023.
Experienced professionals, especially in industry, management, or specialized fields such as bioinformatics and biomanufacturing, can earn more than $100,000. Advanced degrees, particularly PhDs, often carry higher earning potential. Compensation tends to be higher in pharmaceutical and biotechnology companies than in academic or government roles.
Frequently Asked Questions (FAQ)
It's an interdisciplinary field using molecular and genetic tools to manipulate biomolecules for practical applications in health, agriculture, and environment.
Examples include producing recombinant insulin, engineering microbes for biofuels, creating GM crops, CRISPR gene editing, and PCR diagnostic tests.
Roles include Research Scientist, Lab Technician, Bioprocess Engineer, Bioinformatics Specialist, and Quality Control in pharma, food, or environmental sectors.
They design and perform lab experiments using DNA/RNA tools to create new drugs, vaccines, diagnostics, or improve agricultural products.
A biophysicist studies physical forces and structures within biological molecules, using physics tools to understand biological function.
You investigate how biological molecules (like DNA/proteins) function in cells, using lab and computational tools to understand basic processes.
An example is using X-ray crystallography to determine a protein's 3D shape or measuring forces in molecular machines.
Recommended: hepatitis A and B, typhoid, tetanus. Rabies only if engaging in high-risk activities like caving or rural stays.
A: Salaries are respectable and vary; experienced scientists and those with advanced degrees, especially in industry, can earn high incomes.
They work in healthcare, agriculture/food, industrial processes, and environmental applications.
References
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2015). Molecular Biology of the Cell (6th ed.). Garland Science.
Brown, T. A. (2016). Gene Cloning and DNA Analysis: An Introduction (7th ed.). Wiley-Blackwell.
Clark, D. P., & Pazdernik, N. J. (2019). Biotechnology: Applying the Genetic Revolution (2nd ed.). Academic Press.
ScienceDirect. (2017). Synthetic biology: Engineering living systems.
U.S. Bureau of Labor Statistics. (2023). Occupational Outlook Handbook: Biological Technicians and Scientists.
World Health Organization. (2021). Genomics and Precision Medicine.







